Video Description Title
Video Description description
Claudins are present throughout the body, but 2 specific isoforms of CLDN18 are localized to certain tissue types6,7
CLDN18.1
CLDN18.1 is the dominant isoform in normal and malignant lung tissue.
CLDN18.2
CLDN18.2 is the dominant isoform in normal gastric tissue and is often retained in malignant transformation.
Matteo Fassan, MD, PhD
CONFINED IN HEALTHY TISSUE
RETAINED AND EXPOSED IN MALIGNANT TRANSFORMATION
MAINTAINED IN METASTATIC PROGRESSION
The information provided above is based on the current understanding of data.
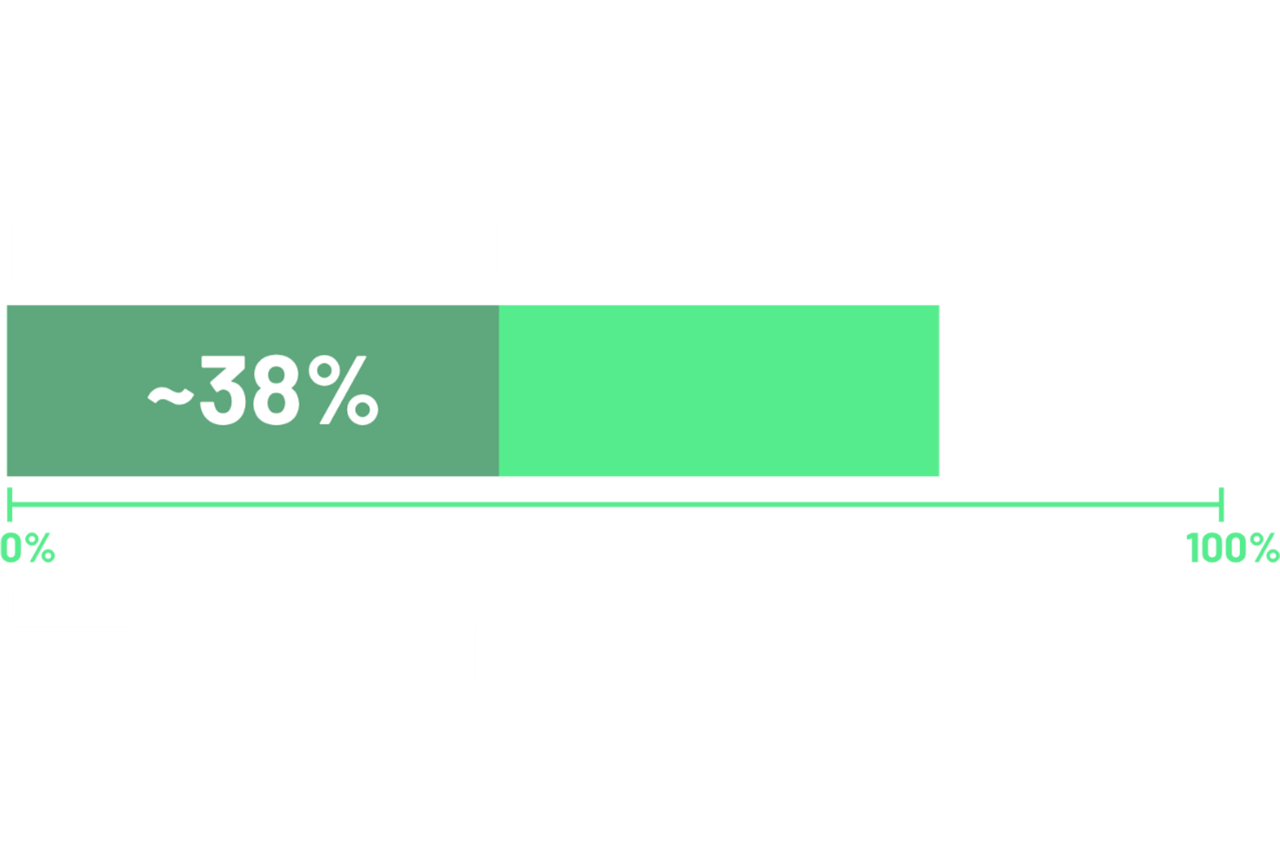
According to 2 recent global studies in patients with locally advanced unresectable or metastatic G/GEJ adenocarcinoma, ~38% of cases demonstrated ≥75% of tumor cells with moderate-to-strong (2+/3+) membranous CLDN18 staining.12,13*
*Data from 2 global randomized phase 3 studies: the first study included 2403 assessable patients, of which 922 were CLDN18.2 positive; and a second study which included 2104 assessable patients, of which 808 were CLDN18.2 positive.12,13
Data from 3 single-institution studies. The first study was undertaken in Padua, Italy and included advanced GCs (n=280) and GECs (n=70).1 The second study was undertaken in 408 Japanese patients with advanced G/GEJ cancers.17 The third study at MD Anderson Cancer Center (MDACC) involved 304 patients.18
*US SEER 21 (excluding IL) 2015-2021, gastric and esophageal cancers, distant stage.19,20
†Locally advanced (stage II and III) and metastatic (stage IV) G/GEJ cancer per TNM staging classification per the American Joint Committee on Cancer (AJCC) Cancer Staging Manual.22


Data in patients with G/GEJ cancers suggest that CLDN18.2 expression demonstrated high concordance between samples of primary and lymph node metastases.10
In a study of 523 primary G/GEJ adenocarcinomas and 135 pair-matched, synchronous nodal metastases10:
As is the case with other biomarkers such as HER2, CLDN18.2 expression may demonstrate variability within a tumor, and this should be taken into account when sampling.10,24
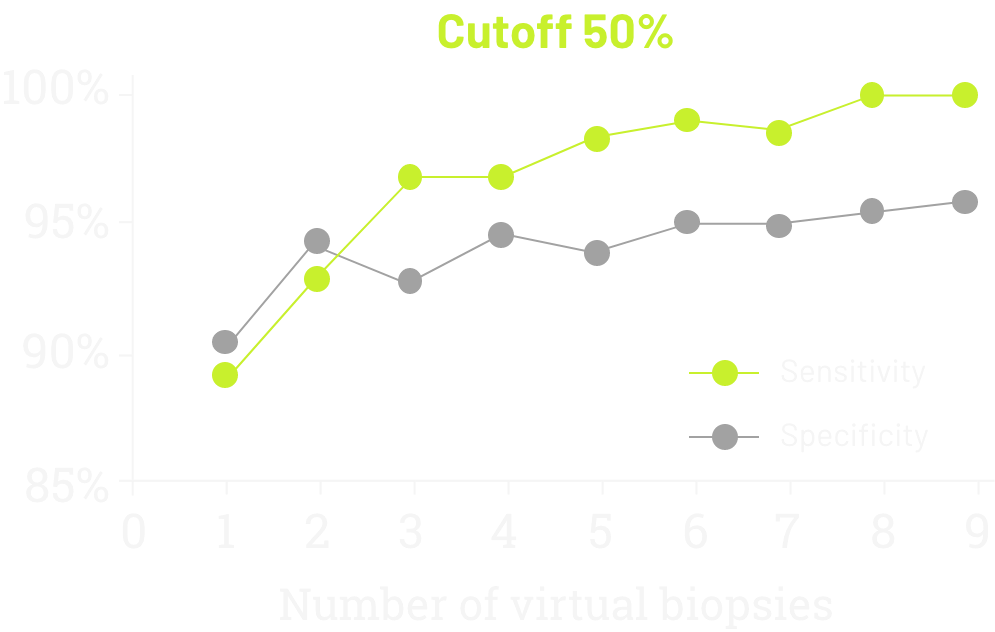
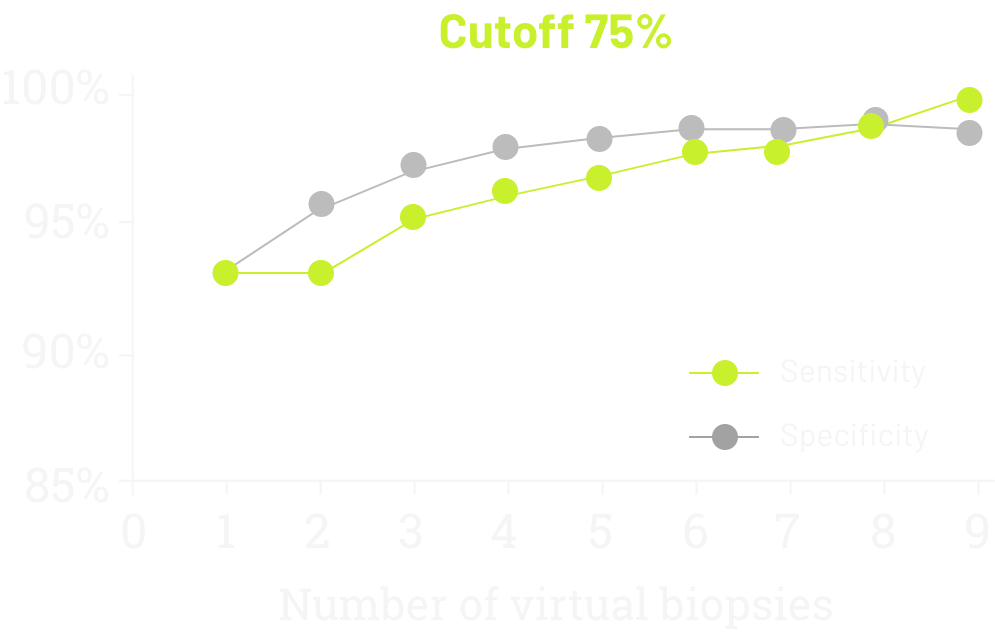
In 2021, data was published demonstrating the sensitivity and specificity of CLDN18 testing according to the number of virtual biopsies scored on 93 surgically treated cases (77 GC, 16 GEJ).1

By signing up, you will receive updates about testing for CLDN18.2. You
may also be contacted by an Astellas representative.
*Required fields
By signing up, you will receive updates about testing for CLDN18.2. You
may also be contacted by an Astellas representative.
*Required fields
References: 1. Pellino A, Brignola S, Riello E, et al. Association of CLDN18 protein expression with clinicopathological features and prognosis in advanced gastric and gastroesophageal junction adenocarcinomas. J Pers Med (Epub) 10-26-2021. 2. Tsukita S, Tanaka H, Tamura A. The claudins: from tight junctions to biological systems. Trends Biochem Sci 2019;44(2):141-52. 3. Hu YJ, Wang YD, Tan FQ, Yang WX. Regulation of paracellular permeability: factors and mechanisms. Mol Biol Rep 2013;40(11):6123-42. 4. Referenced with permission from the NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®) for Gastric Cancer. V.2.2025. © National Comprehensive Cancer Network, Inc.2025. All rights reserved. Accessed April 7, 2025. To view the most recent and complete version of the guidelines, go online to NCCN.org. 5. Referenced with permission from the NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®) for Esophageal and Esophagogastric Junction Cancers. V.3.2025. © National Comprehensive Cancer Network, Inc.2025. All rights reserved. Accessed April 24, 2025. To view the most recent and complete version of the guidelines, go online to NCCN.org. NCCN makes no warranties of any kind whatsoever regarding their content, use or application and disclaims any responsibility for their application or use in any way. 6. Sahin U, Koslowski M, Dhaene K, et al. Claudin-18 splice variant 2 is a pan-cancer target suitable for therapeutic antibody development. Clin Cancer Res 2008;14(23):7624-34. 7. Niimi T, Nagashima K, Ward JM, et al. Claudin-18, a novel downstream target gene for the T/EBP/NKX2.1 homeodomain transcription factor, encodes lung- and stomach-specific isoforms through alternative splicing. Mol Cell Biol 2001;21(21):7380-90. 8. Sahin U, Schuler M, Richly H, et al. Eur J Cancer 2018;100:17-26. 9. Lamouille S, Xu J, Derynck R. Molecular mechanisms of epithelial-mesenchymal transition. Nat Rev Mol Cell Biol 2014;15(3):178–96. 10. Coati I, Lotz G, Fanelli GN, et al. Claudin-18 expression in oesophagogastric adenocarcinomas: a tissue microarray study of 523 molecularly profiled cases. Br J Cancer 2019;121(3):257-63. 11. Rohde C, Yamaguchi R, Mukhina S, Sahin U, Itoh K, Türeci O. Comparison of claudin 18.2 expression in primary tumors and lymph node metastases in Japanese patients with gastric adenocarcinoma. Jpn J Clin Oncol 2019;49(9):870-6. 12. Shitara K, Lordick F, Bang YJ, et al. Lancet 2023;401(10389):1655-68. Errata in: Lancet 2023;402(10398):290; Lancet 2024;403(10421):30. 13. Shah MA, Shitara K, Ajani JA, et al. Nat Med 2023;29(8):2133-41. 14. Van Cutsem E, Bang YJ, Feng-yi F, et al. HER 2 screening data from ToGA: targeting HER2 in gastric and gastroesophageal junction cancer. Gastric Cancer 2015;18(3):476-84. 15. Fuchs CS, Ozguroglu M, Bang YJ, et al. Pembrolizumab versus paclitaxel for previously treated PD-L1-positive advanced gastric or gastroesophageal junction cancer: 2-year update of the randomized phase 3 KEYNOTE-061 trial. Gastric Cancer 2022;25:197-206. 16. Abrahao- Machado LF, Scapulatempo-Neto C. HER2 testing in gastric cancer: an update. World J Gastroenterol 2016;22(19):4619-25. 17. Kubota Y, Kawazoe A, Mishima S, et al. Comprehensive clinical and molecular characterization of claudin 18.2 expression in advanced gastric or gastroesophageal junction cancer. ESMO Open (Epub) 01-05-2023. 18. Waters R, Sewastjanow-Silva M, Yamashita K, et al. Retrospective study of claudin 18 isoform 2 prevalence and prognostic association in gastric and gastroesophageal junction adenocarcinoma. JCO Precis Oncol 2024;8:e2300543. 19. National Cancer Institute. Surveillance, Epidemiology, and End Results Program. Cancer stat facts: esophageal cancer. Accessed June 20, 2025. https://seer.cancer.gov/statfacts/html/esoph.html 20. National Cancer Institute. Surveillance, Epidemiology, and End Results Program. Cancer stat facts: stomach cancer. Accessed June 20, 2025. https://seer.cancer.gov/statfacts/html/stomach.html 21. American Cancer Society. Cancer Facts & Figures 2025. Accessed February 14, 2025. https://www.cancer.org/content/dam/cancer-org/research/cancer-facts-and-statistics/annual-cancer-facts-and-figures/2025/2025-cancer-facts-and-figures-acs.pdf. 22. Gress DM, Edge SB, Greene FL, et al. Principles of cancer staging. In: Amin MB, Edge SB, Greene FL, et al, eds. AJCC Cancer Staging Manual. 8th ed. Chicago, IL: American Joint Committee on Cancer, 2017:3-30. 23. Shitara K, Xu R, Moran D, et al. Presented at the 2023 ASCO Annual Meeting; June 2-6, 2023; Chicago, IL, USA. 24. Grillo F, Fassan M, Sarocchi F, et al. HER2 heterogeneity in gastric/gastroesophageal cancers: from benchside to practice. World J Gastroenterol 2016;22(26):5879-87.