Video Overlay Titlle
Video Overlay description

Why use CLDN18 antibodies for detecting CLDN18.2 in G/GEJ tumor samples?
CLDN18 antibodies can identify both CLDN18 isoforms—CLDN18.1 and CLDN18.2. But when evaluating G/GEJ tumor tissue, staining can be attributed to the presence of CLDN18.2 because2,3:
Sample preparation and preanalytics
Appropriate specimen handling and preparation are essential to ensure the accuracy of biomarker results.1
Takeshi Kuwata, MD, PhD
College of American Pathologists (CAP) Guidelines recommend daily tissue processor maintenance per the manufacturer recommendations, and rigorous quality maintenance of processor fluids, including formalin pH/purity and water contamination of alcohols.1
Cold ischemic time should be limited to ≤60 minutes according to current guidelines.1
CAP Guidelines provide recommendations regarding dimensions and duration involved in sample fixation.1
Tissue should be completely submerged in fixative
Ensure a fixative volume to tissue mass ratio of no less than 4:1, with an optimal ratio of 10:1
Paraffin should be melted at <60°C
Specimen containing sufficient tumor tissue for analysis
As part of stabilization, tissue should be fixed in 10% neutral phosphate-buffered formalin (pH 7.0) for at least 6 hours and no longer than 24 to 36 hours
If the tissue has high fat content, fixation may require up to 48 hours
It is important to optimize pre-analytical variables to minimize staining artifacts, which can interfere with accurate scoring.
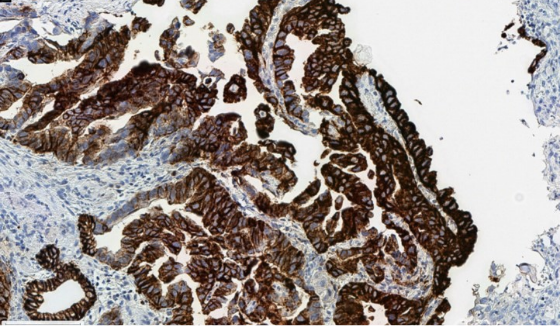
Cytoplasmic blushing due to suboptimal fixation, which can interfere with accurate membranous scoring.
Routinely processed, formalin-fixed, paraffin-embedded (FFPE) tissues are suitable for use with IHC testing
Specimens that are fine-needle aspirate (FNA), cytology specimens or metastatic bone lesions do not qualify for CLDN18 staining
Tissue sections can be cut at 3 μm-6 μm*
Before staining, the cut slides should be dried completely either at room temperature (air dried) or by offline baking (baked in oven) at 60°C for 60 minutes*
To ensure integrity of specimens, storage areas should be:
Dry
Pest-free
Room temperature (18°C to 25°C)
A number of assays and antibodies are available to assess CLDN18.2 expression. The list below is not exhaustive. The appropriate test should be used when guiding clinical decision-making.2,4-6
| Name | Company | Clone Name | Clonality | Host | Isotype | Use |
|---|---|---|---|---|---|---|
| VENTANA® CLDN18 (43-14A) RxDx Assay® | Ventana Medical Systems a member of the Roche Group | 43-14A | Monoclonal | Mouse | IgG2b | CDx/IVD |
| PathPlusTM CLDN18/Claudin 18 Antibody | LSBio an Absolute Biotech Company | LS-B16145 | Monoclonal | Mouse | IgG | RUO |
| Recombinant Anti-Claudin 18 antibody (43- 14A) | Abcam | 43-14A | Monoclonal | Mouse | IgG2b | RUO |
| Claudin-18 Antibody | Novus Biologicals | NBP2-32002 | Polyclonal | Rabbit | IgG | RUO |
Christoph Röcken, MD
In the global RING study, assessing the reproducibility and comparability of three CLDN18 antibodies and IHC staining platforms across a cohort of 27 global laboratories where sensitivity, specificity, accuracy, and precision were analyzed7*†:
*Antibodies in the study comprised the VENTANA CLDN18 (43-14A) IVD Assay from Roche Tissue Diagnostics, the PathPlus™ CLDN18 Antibody from LSBio, and the Claudin-18 Antibody from Novus Biologicals. Platforms comprised BenchMark ULTRA, Dako Autostainer, and Leica Bond.7
†Consensus reference scores from all antibodies for each sample were determined by central pathology review. CLDN18.2 positivity was defined with a threshold of ≥75% of tumor cells expressing membranous CLDN18 with moderate-to-strong (≥2+) staining intensity. Accordingly, participating pathologists were required to submit a binary positive/negative call as well as an estimation of the percent of cells stained. Laboratory-submitted IHC scores were compared to the reference consensus score and considered discordant if the positive/negative binary result differed. Statistical analysis was performed for comparison, and an acceptance criteria of 85% (≥0.85) was applied.7
Appropriate controls are essential for the detection of CLDN18.2 in G/GEJ tumor samples. Here are some key points on their selection and use.2,8
Guidelines recommend that laboratories validate and/or verify immunohistochemical tests before placing them into clinical service and should include positive, negative, and borderline tissue, reflecting the intended use of the assay.8
Tissue controls are commercially available through various providers.

NCCN Guidelines® recommend testing via IHC for actionable biomarkers including CLDN18.2, HER2, and PD-L1 at the time of diagnosis if advanced/metastatic disease is suspected.10,11
Biomarkers should be tested concurrently to allow for timely reporting of results.9
Click below for full guidelines.
It’s critical that pathologists advocate for testing at diagnosis for all prevalent, actionable biomarkers.
Matteo Fassan, MD, PhD

Implementation of a reflex ordered testing strategy at the time of pathologic diagnosis:

Additionally, reflex testing can13:

CAP, College of American Pathologists; CDx=companion diagnostic; CLDN18, claudin 18; CLDN18.1, claudin 18 isoform 1; CLDN18.2, claudin 18 isoform 2; G/GEJ, gastric/gastroesophageal junction; IHC, immunohistochemistry; IVD, in vitro diagnostic; RUO=research use only.
References: 1. Compton CC, Robb JA, Anderson MW, et al. Preanalytics and precision pathology: pathology practices to ensure molecular integrity of cancer patient biospecimens for precision medicine. Arch Pathol Lab Med 2019;143(11):1346-63. 2. VENTANA CLDN18 (43-14A) assay [package insert]. Mannheim, Germany: Roche Diagnostics GmbH. 3. Sahin U, Koslowski M, Dhaene K, et al. Claudin-18 splice variant 2 is a pan-cancer target suitable for therapeutic antibody development. Clin Cancer Res 2008;14(23):7624-34. 4. LSBio. PathPlus CLDN18 Claudin 18 Monoclonal Antibody Mouse IHC. Accessed May 15, 2024. https://www.lsbio.com/pathplus-antibodies/pathplus-cldn18-antibody-claudin-18-antibody-ihc-ls-b16145/789855. 5. Abcam. Product datasheet. Anti-Claudin18 antibody [43-14A] ab314690. Accessed May 15, 2024. https://www.abcam.com/products/primary-antibodies/claudin18-antibody-43-14a-ab314690.html. 6. Novusbio. Product datasheet. Claudin-18 Antibody NBP2-32002. Accessed May 15, 2024. https://www.novusbio.com/products/claudin-18-antibody_nbp2-32002. 7. Jasani B, Taniere P, Schildhaus HU, et al. Global ring study to investigate the comparability of total assay performance of commercial claudin 18 antibodies for evaluation in gastric cancer Lab Invest 2024;104(1):100284. 8. Fitzgibbons PL, Bradley LA, Fatheree LA, et al. Principles of analytic validation of immunohistochemical assays: guideline from the College of American Pathologists Pathology and Laboratory Quality Center. Arch Pathol Lab Med 2014;138(11):1432-1443. 9. Piening B, Bapat B, Weerasinghe RK, et al. Improved outcomes from reflex comprehensive genomic profiling-guided precision therapeutic selection across a major US healthcare system [Abstract 6622]. J Clin Oncol 2023;41(Suppl 16). 10. Referenced with permission from the NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®) for Gastric Cancer. V.2.2025. © National Comprehensive Cancer Network, Inc.2025. All rights reserved. Accessed April 7, 2025. To view the most recent and complete version of the guidelines, go online to NCCN.org. 11. Referenced with permission from the NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®) for Esophageal and Esophagogastric Junction Cancers. V.3.2025. © National Comprehensive Cancer Network, Inc.2025. All rights reserved. Accessed April 24, 2025. To view the most recent and complete version of the guidelines, go online to NCCN.org. NCCN makes no warranties of any kind whatsoever regarding their content, use or application and disclaims any responsibility for their application or use in any way. 12. Anand K, Phung TL, Bernicker EH, Cagle PT, Olsen RJ, Thomas JS. Clinical utility of reflex ordered testing for molecular biomarkers in lung adenocarcinoma. Clin Lung Cancer 2020;21(5):437-42. 13. Gosney JR, Paz-Ares L, Janne P, et al. Pathologist-initiated reflex testing for biomarkers in non-small-cell lung cancer: expert consensus on the rationale and considerations for implementation. ESMO Open 2023;8(4);1-8.